
The immune system, a tightly knit network of cells, tissues and biochemicals that they secrete, defends our body against fungi, bacteria, viruses, and other invaders. But cancer and some infections often find ways to hide from the immune system or block its ability to fight. The 5th international Else-Kröner-Symposium “Translational Immunology – From Target to Therapy V” took place in Würzburg to exchange the newest research and clinical data on novel approaches to foster the immune system to prevent and treat human diseases.
This year’s symposium from April 12-13, 2018, was organized by our young physician-scientists in training of the Else-Kröner-Forschungskolleg for Interdisciplinary Translational Immunology. They are ten selected and associated fellows from different medical disciplines (cardiology, dermatology, gynecology, hematology-oncology, internal medicine, urology and nuclear-medicine). On top of their training in their respective medical fields Else-Kröner-fellows participate in a structured program for translational immunological research. As part of their program they are also organizing an annual symposium, “Translational Immunology – From Target to Therapy”. At this event supported by the Else-Kröner-Fresenius-Stiftung there is the opportunity to listen to leaders in translational immunology research from all over the world and to present and discuss their work with these international experts in immunodiagnostics and -therapy.

Immunotherapy tries to help the immune system recognize cancer or infections as a threat, and attack it. Although many approaches to harness our immune system are investigated two promising types of immunotherapy have entered clinical cancer therapy. One creates a new, individualized treatment for each patient by removing some of the person’s immune cells, altering them genetically to kill cancer and then infusing them back into the bloodstream. Although this treatment has so far been still limited to hematological malignancies, it has resulted in long remissions in a few hundred children and adults with deadly forms of leukemia or lymphoma for whom standard treatments had failed. The second approach, which is now being widely explored in all forms of cancer, involves mass-produced therapeutic antibodies that do not have to be tailored to each patient. These biological drugs foster immune cells to fight cancer by blocking a mechanism — called a checkpoint — that cancer cells abuse to shut down the immune system. Beside immune checkpoints and genetically engineered T cells, the symposium covered many other topics, from autoimmune disease to neuro-immunology.
Again, this year’s Else-Kröner-Symposium provided a great platform to listen to and interact with international leaders in cancer immunotherapy, inflammation, autoimmune and infectious diseases. The list of speakers included Philipp Beckhove (Regensburg), Mark Coles (Oxford), Angus Dalgleish (London), Wilfried Ellmeier (Wien), Manuel Friese (Hamburg), Thomas Gebhardt (Melbourne), Audrey Gérard (Oxford), Michael Hölzel (Bonn), Alexander Kerster (Rehovot), Christopher Klebanoff (New York), Thomas Korn (München), Jens Stein (Bern), Viktor Umansky (Heidelberg), Sjoerd van der Burg (Leiden), Hans van Eenenaam (Nijmegen) and Robert Zeiser (Freiburg). Again this year one physician scientist in training and one PhD researcher were awarded with a poster prize. This year’s awardees are Dr. Tanja Stüber from the Department of Obstetrics and Gynecology and Dr. Duc Dung Le from the Beilhack lab – Congratulations to our young scientists!
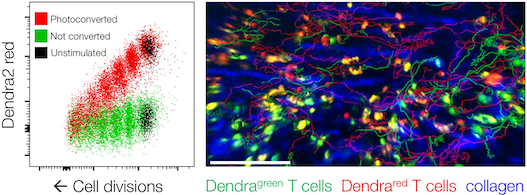
 Read more about our new research article on how a powerful microscopy technique helps scientists to find a needle in a haystack – only 2 micrometer thick fungal spores (50 times thinner than a hair) in an entire lung to learn about the ensuing immune response.
Read more about our new research article on how a powerful microscopy technique helps scientists to find a needle in a haystack – only 2 micrometer thick fungal spores (50 times thinner than a hair) in an entire lung to learn about the ensuing immune response.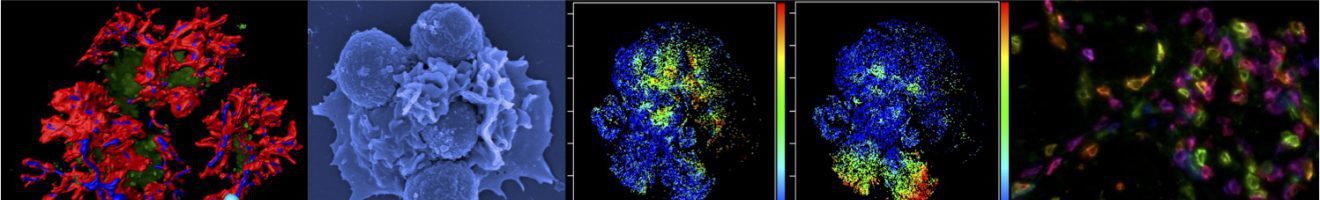
 On November 16, 2019, the foundation
On November 16, 2019, the foundation 

 The annual meeting of the immunology programs from Würzburg, Erlangen and Tübingen Universities took place in Obertrubach, Franconia. This year’s meeting was organized by Hans-Martin Jäck and his team from the University of Erlangen.
The annual meeting of the immunology programs from Würzburg, Erlangen and Tübingen Universities took place in Obertrubach, Franconia. This year’s meeting was organized by Hans-Martin Jäck and his team from the University of Erlangen.








